Fighting superbugs with peptides found in human body
Superbugs, or bacteria resistant to antibiotics, are becoming a growing health threat worldwide. It is estimated that if nothing is done to fight them, superbugs could kill 10 million people a year by 2050. Researchers around the world are racing to find new treatments to stop the spread of the drug-resistant bacterial strains.
Among them is Kaori Sugihara, an associate professor at the Institute of Industrial Science. She studies antimicrobial peptides (AMPs), naturally occurring molecules composed of a small chain of amino acids and produced by the human and animal body when infected by bacteria. These peptides are seen as promising candidates for fighting drug-resistant pathogens.
“There are hundreds of different AMPs in our bodies. The peptides not only kill superbugs, but they also impede the development of drug resistance,” Sugihara said. “The development of new antibiotics and the emergence of antibiotic resistance that ensues are locked in a game of cat and mouse. It’s been reported that in extreme cases, novel antibiotic-resistant bacteria can emerge just hours after a new drug has been developed. In that regard, AMPs are said to buy us decades of time.”
AMPs attack bacteria by attaching to lipids in the bacteria cell membranes, punching holes and killing the microbes. The problem, however, is that they can also attack and damage healthy human body cells. Peptide-based antibiotics such as polymyxin, for example, come with possible side effects that could damage the kidney.
In 2020, Sugihara discovered that mixing two major AMPs — LL37 and HNP1 — increases toxicity against drug-resistant bacteria, thereby being effective in killing the bad germs, while reducing toxicity to human cells and protecting them. Naming this phenomenon the double cooperative effect, Sugihara is currently working to find out the mechanism behind it.
“How does it work? Are there other peptide combinations that produce a similar effect? We’ve been studying to find answers to these questions,” Sugihara explained. “If the double cooperative effect is indeed real and something we can control, we may be able to develop new medicines that are highly antibacterial and have fewer side effects simply by mixing two types of peptides.”
From basic research on lipids to developing tools

Sugihara studied theoretical physics at the Faculty of Science and Technology at Keio University in Tokyo, before moving on to semiconductor physics for her master’s degree at the University of Tokyo’s Graduate School of Engineering. After completing the master’s degree, she entered a doctoral course at the Swiss Federal Institute of Technology (ETH) Zurich. There, with the hope of expanding the scope of her research into biology and medicine, Sugihara switched her field to biophysical engineering.
“My initial research was to develop devices for analyzing biomolecules (organic molecules, including proteins, carbohydrates and lipids, which are produced by living organisms). But as I began to talk more with biologists who use the instruments, I started to have this growing desire to use the tools myself to conduct research,” she said. “That led me to gradually shift my focus from developing devices to using them for analyzing biomolecules.”
Since her doctoral studies, Sugihara has focused on lipids, biomolecules that are components of cell membranes. Together with proteins embedded in cell membranes, lipids regulate critical functions, such as immunity, the senses and muscle activity. They have both hydrophilic (water-loving) and hydrophobic (water-hating) properties, much like soap. Thus, lipids share structural similarities with soap and the biomolecules can change shape depending on their environment. Sugihara has been studying ways to manipulate this phenomenon, called self-assembly, at the nanoscale level for practical applications.
One such application is as a biosensor — a device using biological materials (lipids in this case) to detect the presence of chemicals in a substance. Some lipids transform into material called mechanochromic polymers when exposed to ultraviolet light. These polymers emit light when mechanical force is applied. Using this property, Sugihara aims to develop sensors capable of predicting peptides’ capacity, such as their strength against superbugs.
“In order to develop antibiotics using peptides, we need to identify powerful candidates from countless combinations,” she said. “If we can develop this tool, we could use it to screen for peptides that have similar functions to those that are currently known.”
To further accelerate her research, Sugihara has also begun studying artificial intelligence to develop machine learning models. Her ultimate goal is drug development.
“Once we reach a certain point, we’ll need to find collaborators to move forward. That said, given that it takes a very long time to develop a drug, if I can get there within my lifetime, that would be an achievement,” Sugihara said.
Recharging static electricity in masks
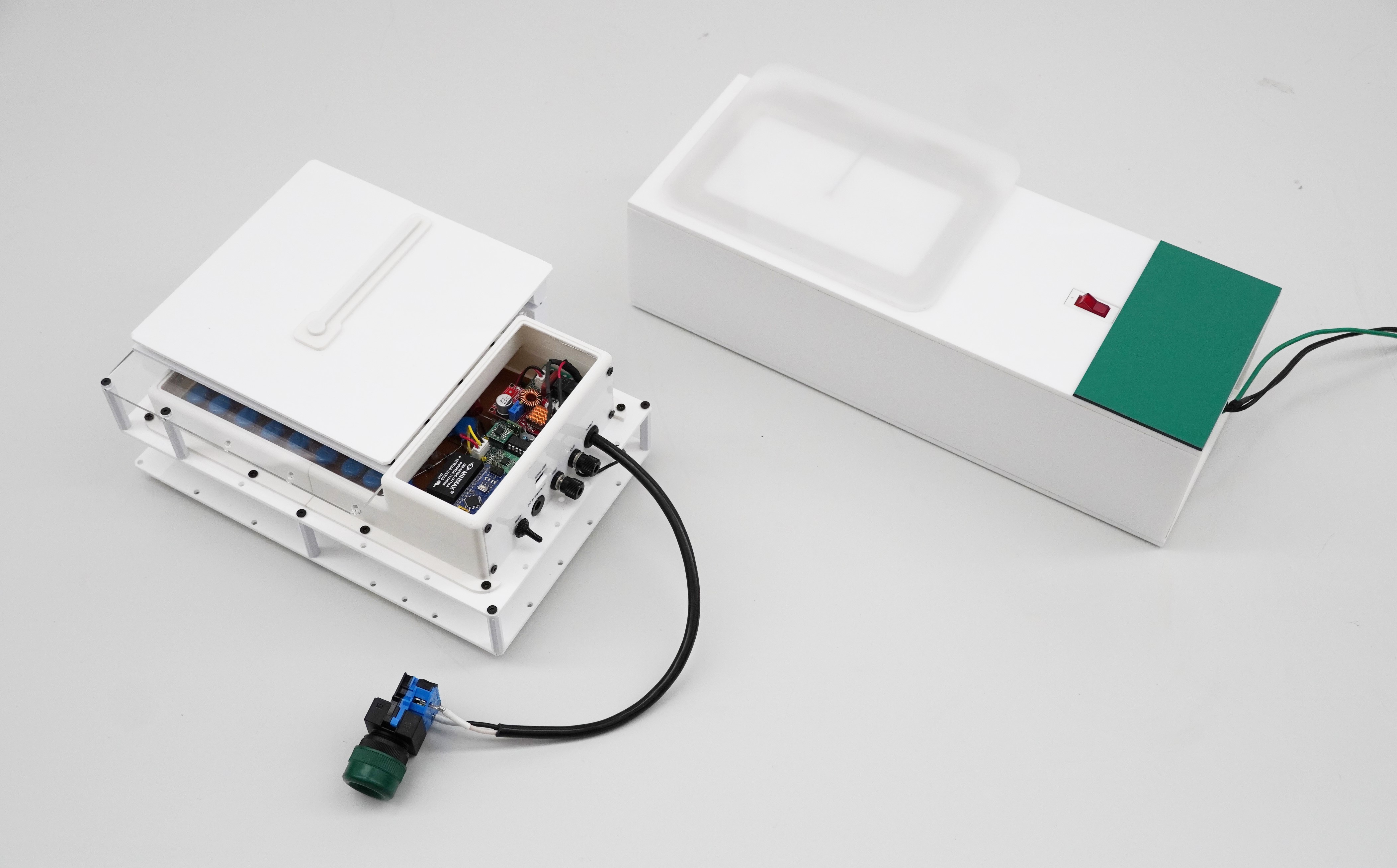
Alongside her lipids-related research, Sugihara has been developing a device, called a mask charger, which restores filtering efficiency to face masks, since she joined UTokyo in 2020 — amid the COVID-19 pandemic when the world faced a mask shortage. Alarmed by media reports that some hospitals were washing and reusing disposable surgical masks due to the shortage, Sugihara launched the research project.
Nonwoven masks not only physically block particles but also use static electricity in their polypropylene mesh to capture tiny aerosols that would otherwise sneak through. Humidity, however, quickly weakens the static charge. The masks’ filtering ability, for example, is rapidly lost during Japan’s humid summer. The mask charger Sugihara and her team developed in 2023 recharges the lost static electricity.
The device is based on an electrical circuit called a Cockcroft-Walton’s voltage multiplier. By placing a mask on the charger, closing the lid and turning on the switch, the mask can be recharged in just a few seconds. Sugihara is currently developing a second model with improved safety features in collaboration with Hashimoto Cloth Corp. The partners plan to commercialize their product by 2026. Given that the mask shortage is no longer a public health problem, their current target customers are municipalities and factories.
“Municipalities often store huge numbers of masks for emergencies. But masks have expiration dates. Every few years, huge quantities of masks are discarded worldwide and replaced with newly purchased ones. That’s costly and becomes a burden on the environment,” she said. “If the only problem standing in the way is the loss of static electricity, then we can recharge the masks and restore their function. That’s what we are aiming for.”
Sugihara said the most exciting moment as a researcher is when results defy expectations.
“When you get results that align with your expectations, it means you haven’t learned anything new. But when the data are contradictory, it shows your assumptions were wrong. Figuring out why can lead to a new discovery. I want to continue embracing such ‘failures,’” she said.